How does ionic radius change across a period?
1 Answer
Atomic size DECREASES across a Period, from left to right as we face the Table........
Explanation:
But ionic size INCREASES across a Period, from left to right......
How do we reconcile the two processes.......?
Metals are electron-rich materials, and come from the left hand side of the Periodic Table. These are REDUCING materials, that tend to form cations,
The cation has a MUCH smaller radius than the parent atom, especially for say the alkali metals, where the valence electron occupies the outermost shell, and the radius of the resultant cation is MUCH smaller.
On the other hand, non-metals, fluorine, oxygen, etc. are OXIDIZING materials, that tend to accept electron density.......
Because the electron joins a valence shell to fully occupy it, the ionic radius of the anion is GREATER than that of the parent atom.
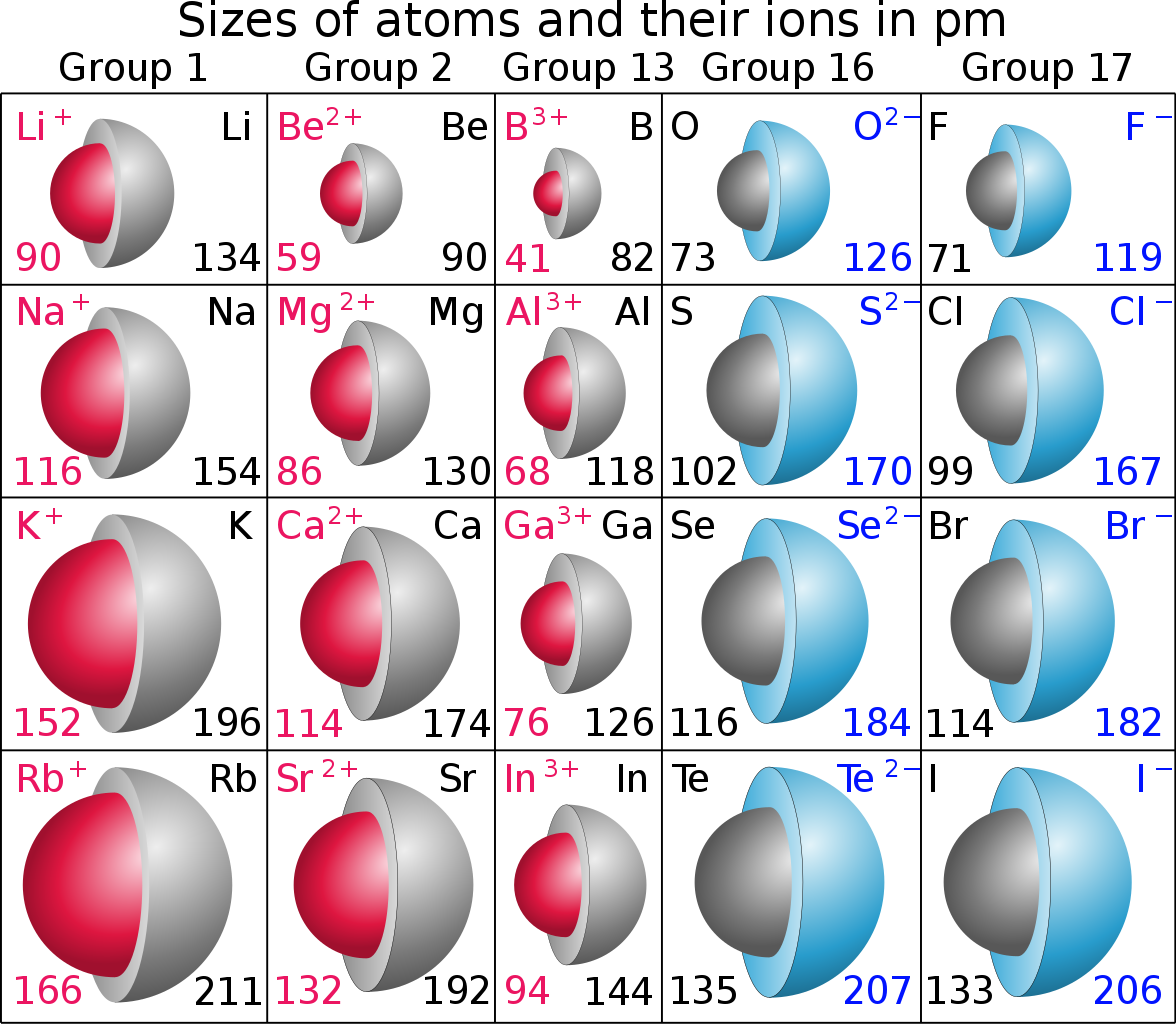
Do the data given in the illustration support what I say?

