How does ionic radius increase?
1 Answer
Of course it depends on what ion you form...........
Explanation:
Reduction leads to an increase in the radius of the resultant ion, WITH RESPECT TO THE PARENT ATOM.....
And conversely, oxidation leads to a decrease in the radius of the resultant ion, WITH RESPECT TO THE PARENT ATOM.......
Well let's see if we can find some data........
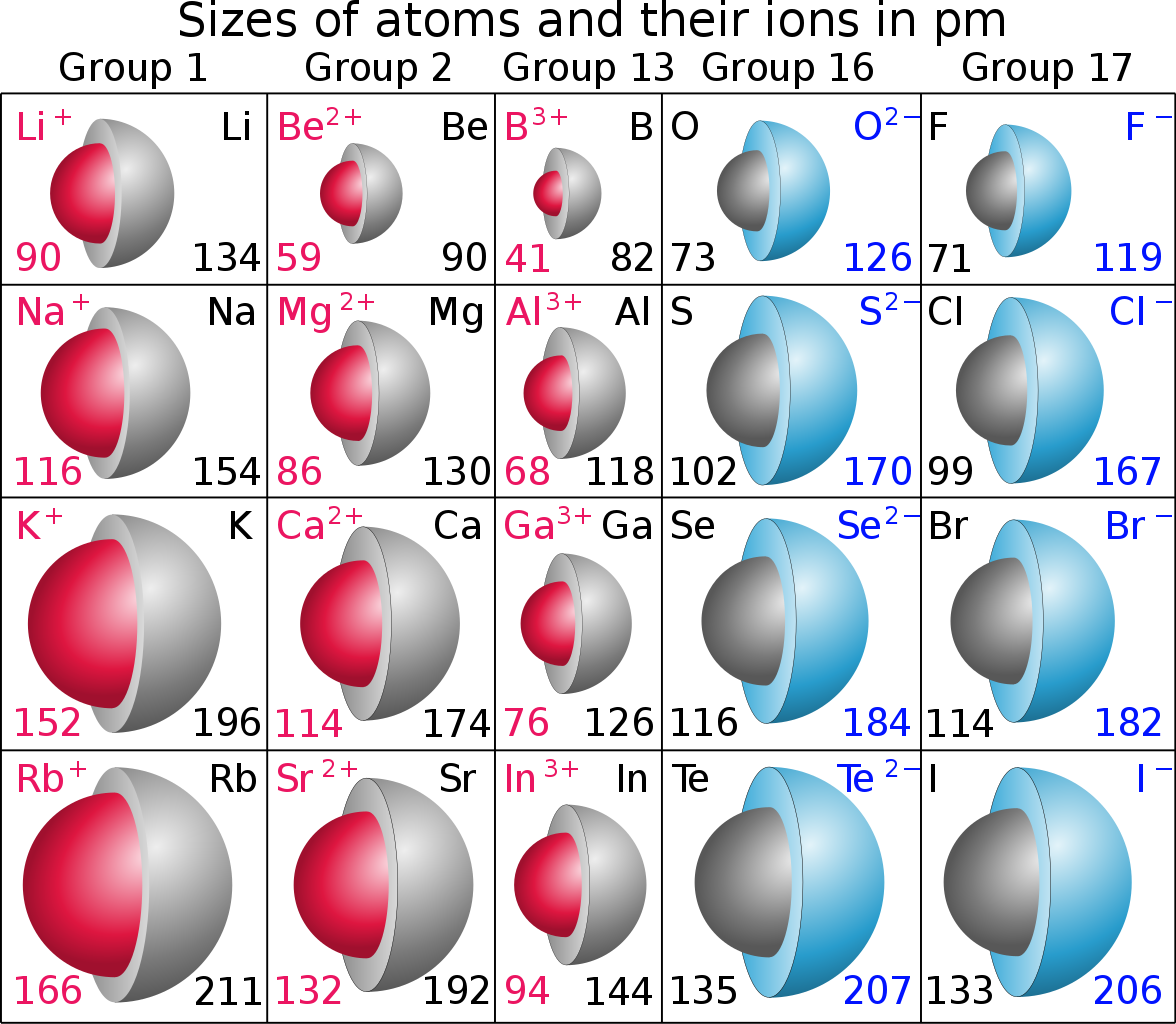
Do these data support the given argument? It should, because atomic size is measured on the basis of the electronic radius of the valence electrons. And if we remove this valence electrons, by oxidation of say sodium or potassium or lithium to form
And upon reduction (of a non-metal), the added electrons are perforce directed to the valence shell, and thus, because electrons repel each other, the anionic radius is increased quite markedly with respect to the parent atom. The diagram uses units of

