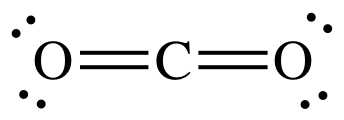How many single bonds are in CO_2?
1 Answer
May 27, 2016
No single bond
Explanation:
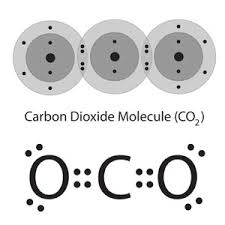
The carbon atom has 4 electrons in its outermost shell (valence shell)
and Oxygen atom has 6 electrons in its outermost shell (valence shell)
One Carbon atom shares its 4 electrons contributing 2 electrons with each of 2 O atoms forming two double bonds as shown below.
So no single bond is present in