What happens to a neutral nonmetal atom in order for the octet rule to be satisfied?
1 Answer
It either gains one or more electrons to form a charged monoatomic ion or share one or more pairs of electrons with another atom.
Explanation:
The nonmetal atom becomes an ion
Nonmetals can receive electrons from a complete electron transfer to form negatively-charged ions. For example, a neutral chlorine atom might gain one electron to form a chloride
The electron added in this process would fill the empty
"Cl" :1s^2 2s^2 2p^2 ul(3s^color(navy)(2) 3p^color(purple)(5)) color(white)(l) color(grey)("incomplete valence shell") "Cl"^(-) :1s^2 2s^2 2p^2 ul(3s^color(navy)(2) 3p^color(navy)(6)) color(white)(l) color(grey)("extra electron completes the octet")
The nonmetal atom forms covalent bonds with other atoms
Alternatively, a nonmetal atom might share one or more pairs of electrons with another atom to achieve an octet. Atomic orbitals of the two atoms "overlap" in a way that valence electrons from one atom fill vacancies in electron orbitals of the other.
For example, a hydrogen atom joins a chlorine atom to form a hydrogen chloride molecule.
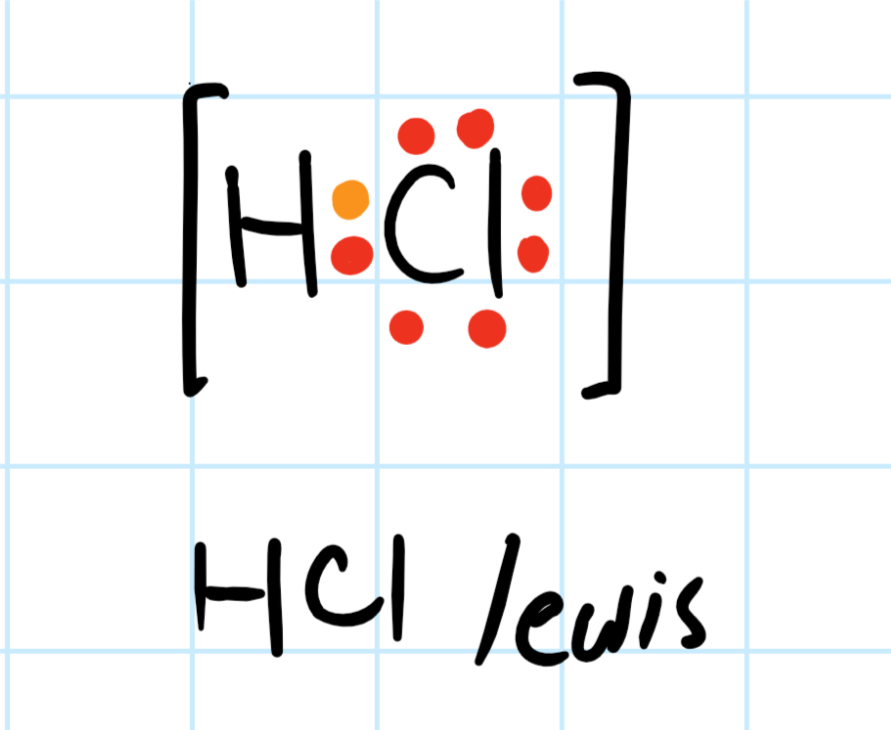 https://socratic.org/questions/what-is-the-lewis-electron-dot-structure-for-a-molecule-of-hydrogen-chloride
https://socratic.org/questions/what-is-the-lewis-electron-dot-structure-for-a-molecule-of-hydrogen-chloride
The chlorine atom has now attained an octet. (Keep in mind that it takes only two valence electrons for a hydrogen atom to achieve the electron configuration of noble gas helium

