What is a free-radical reaction?
1 Answer
Nov 5, 2015
A chemical reaction involving free radicals is a free radical reaction.
Explanation:
Maximum free radical reactions consist three chain steps: Chain initiation, chain propagation and chain termination step.
Radical reactions are very often initiated by light and are not dependent on polarity of the reaction medium.
Halogenation of alkanes is a good example of free radical reaction, esp. the chlorination of methane where chloroform(trichloromethane) and tetrachloromethane are formed. The UV light initiates the reaction and it also follows the three steps. 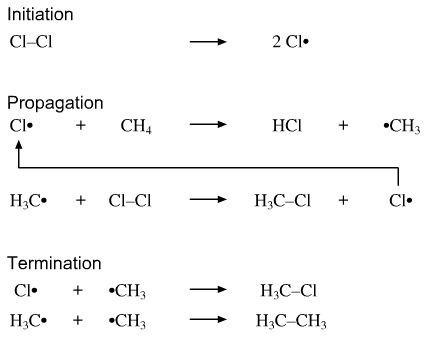 www.meta-synthesis.com
www.meta-synthesis.com

