What is the base catalyzed amide hydrolysis mechanism?
1 Answer
To make this very simple, I will try not to go into details and explain the basics.
Explanation:
Amides are molecules that can form from a carboxylic acid and an amine group. In our body, when two amino acids come together to react, they form an amide bond, releasing
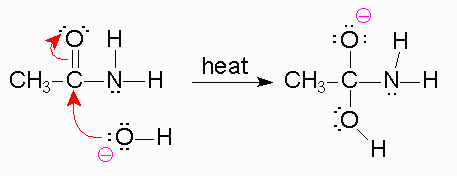 https://chemistry.boisestate.edu/richardbanks/organic/amidehydrolysisbasictutorial2.htm
https://chemistry.boisestate.edu/richardbanks/organic/amidehydrolysisbasictutorial2.htm
To begin, we know that in the first step, a strong base is going to be used like
Now, when the bond forms between the
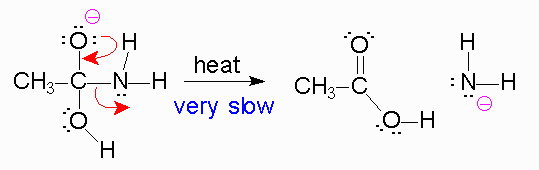 https://chemistry.boisestate.edu/richardbanks/organic/amidehydrolysisbasictutorial3.htm
https://chemistry.boisestate.edu/richardbanks/organic/amidehydrolysisbasictutorial3.htm
*The reason the amine is a poor leaving group is because the amine is a strong base, and strong bases are very unstable. Leaving groups are based off their stability in solution, like the halides, tosylate,
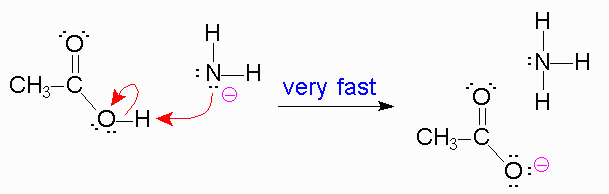 https://chemistry.boisestate.edu/richardbanks/organic/amidehydrolysisbasictutorial4.htm
https://chemistry.boisestate.edu/richardbanks/organic/amidehydrolysisbasictutorial4.htm


