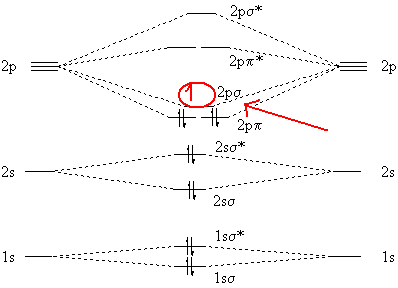
What is the molecular orbital diagram for #C_2^-#?
The molecular orbital diagram for #C_2#
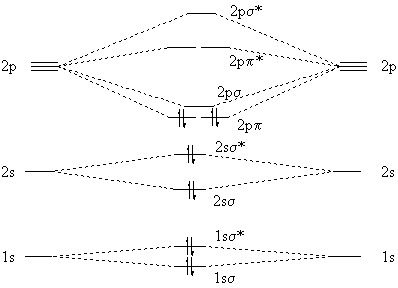
The molecular orbital diagram for

1 Answer
Here's what I got.
Explanation:
The problem provides you with the MO diagram for the
You need to add an electron and not remove one because of the overall negative charge that exists on the molecule. As you know, a neutral carbon atom has a total of
This, of course, implies that a
#2 xx "6 e"^(-) = "12 e"^(-)#
It thus follows that the
#"12 e"^(-) + "1 e"^(-) = "13 e"^(-)#
So, where would this extra electron go in terms of molecular orbitals?
It will be added to the lowest energy unoccupied molecular orbital, or lowest unoccupied molecular orbital,
As you can see in the diagram, the two
The lowest energy unoccupied molecular orbital is
The electron configuration of the neutral
#"C"_ 2: (1s_ (sigma))^2 (1s_ (sigma)^"*")^2 (2s_ (sigma))^2 (2s_ (sigma)^"*")^2 (2p_ (pi))^4#
The electron configuration of the
#"C"_ 2^(-): (1s_ (sigma))^2 (1s_ (sigma)^"*")^2 (2s_ (sigma))^2 (2s_ (sigma)^"*")^2 (2p_ (pi))^4 color(red)((2p_ (sigma))^1#
Notice that because the extra electron is added to a bonding MO, the bond order of the
#"B.O"_ ("C"_ 2^(-)) > "B.O"_ ("C"_ 2)# I won't show the calculation here because I'm not sure you're familiar with bond orders yet
Also, an unpaired electron will make the
On the other hand, the neutral