What is the SN2 reaction mechanism?
1 Answer
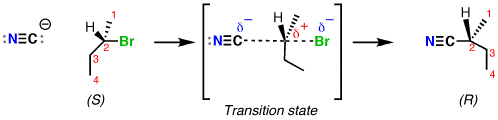
As we will learn, it will also invert the stereochemistry of the substrate (the molecule acted upon).
THE REACTION
An example is the following:
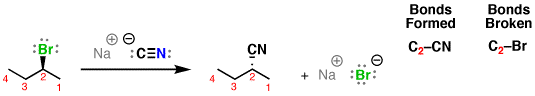
The substrate is 2-bromobutane, and the nucleophile is cyanide (
#"CN"^(-)# ). The electrophilic carbon is carbon-2.
Both the strength of the nucleophile and bulkiness of the substrate, as well as their concentrations, dictate the rate of reaction.
In addition, polar aprotic solvents dissolve the nucleophile well, and also do not protonate it, letting it do its job as a nucleophile.
You can probably see that this isn't going to give a great yield of an
THE MECHANISM
The mechanism for an
-
For this mechanism, the
#"N"-="C":^(-)# simply approaches carbon-2 from behind, and the three groups on carbon-2 "flip" backwards. -
Then,
#"N"-="C":^(-)# attaches by donating a pair of electrons to carbon-2's antibonding orbital, and#"Br"^(-)# leaves after retracting its bonding pair of electrons.
(The planar transition state illustrates a halfway-point between the initial and final stereochemistry.)
Notice how the product is a single stereoisomer, and is NOT a racemic mixture (equal ratio of stereoisomers).
Unlike