Why are tertiary alcohols very inactive in acid catalyzed hydro-alkoxy additions?
1 Answer
Because they are bulky and sterically-hindered.
I've drawn here the mechanism for an acid-catalyzed hydro-alkoxy addition using just ethanol and strong acid:
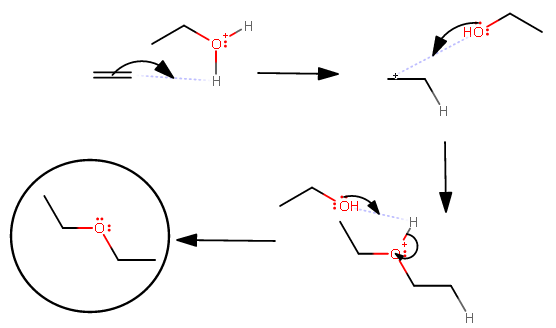
You can plainly see that the alcohol has to end up acting as the nucleophile, but tert-butanol...
...is too bulky and the reaction would take a very long time to proceed. The alcohol would have to move around slowly until it finds the right orientation with the cationic intermediate for the backside attack. By the time it finds the right orientation so that orbital blockage is not an interference, the good leaving group
tert-butanol is better off doing acid-base reactions at a high temperature, such as the first step in an elimination reaction.

