Why do SN2 reactions prefer aprotic solvents?
1 Answer
Aprotic solvents make nucleophiles more nucleophilic.
Explanation:
Let's first look at polar protic solvents.

Protic solvents can H-bond to nucleophiles.
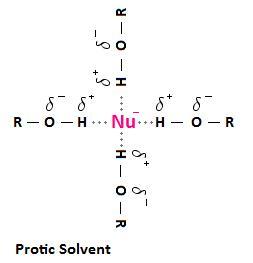
They form a solvation shell around the nucleophile. This shell hinders the nucleophile from attacking the substrate.
Nucleophiles are
Here are some typical polar aprotic solvents.
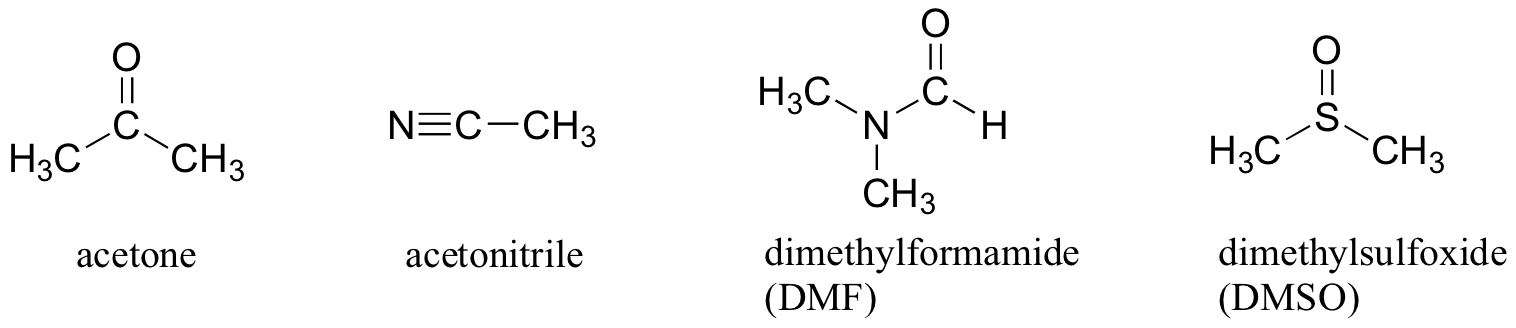
The negative ends of the dipoles are the
They point away from the body of the molecule. This makes it easy for them to solvate cations.
The positive ends are the
They are "buried" in the body of the molecule.
The
The nucleophiles are almost unsolvated, so it is much easier for them to attack the substrate.
Nucleophiles are
So,

