Why are antibonding orbitals higher in energy?
1 Answer
Jul 20, 2014
Antibonding orbitals are higher in energy because there is less electron density between the two nuclei.
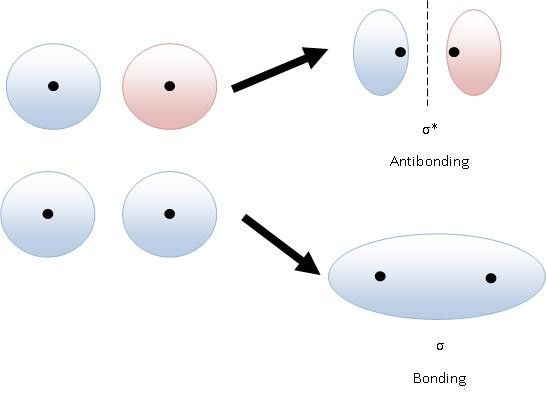
Electrons are at their lowest energy when they are between the two positive nuclei.
It takes energy to pull an electron away from a nucleus. Thus, when the electrons in an antibonding orbital spend less time between the two nuclei, they are at a higher energy level.

