What is hyperconjugation?
1 Answer
Hyperconjugation is the overlap of a

Only electrons in bonds that are
This overlap of orbitals and delocalization of electrons increases the stability of the system
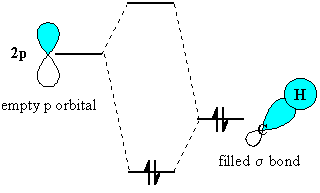
Hyperconjugation explains the relative stability of carbocations:
(CH₃)₃C⁺ > (CH₃)₂CH⁺ > (CH₃)CH₂⁺ > CH₃⁺
The C–C
As it does so, the three C–H
The more adjacent C-H bonds there are, the larger the hyperconjugation stabilization is.
An ethyl cation has three C-H
This agrees with the observed order of stability.
Here’s a video that explains hyperconjugation.


