Would 2-chloropropane or 1-chloro-2,2-dimethylpropane undergo substitution faster with HC≡C−, Na+?
1 Answer
2-Chloropropane will undergo substitution faster than 1-chloro-2,2-dimethylpropane.
The acetylide anion, HC≡C⁻, is the conjugate base of the extremely weak acid, acetylene.
So it is both a powerful nucleophile and an extremely strong base — about a billion times as strong as hydroxide ion.
It undergoes
But acetylide ion is such a strong base that it undergoes mainly
And it undergoes only
An
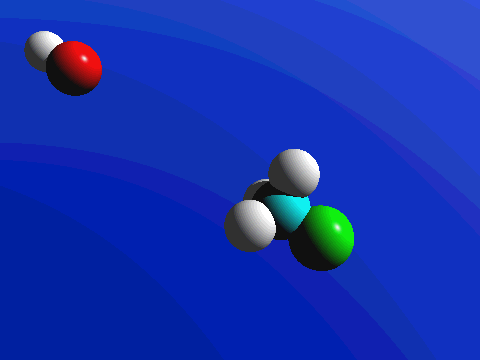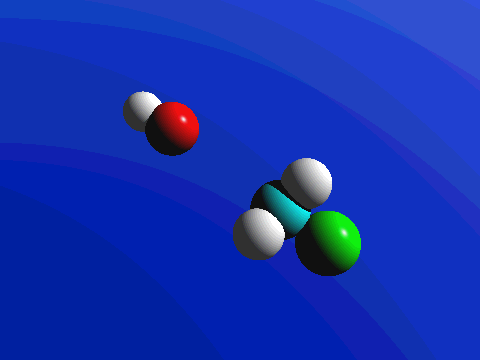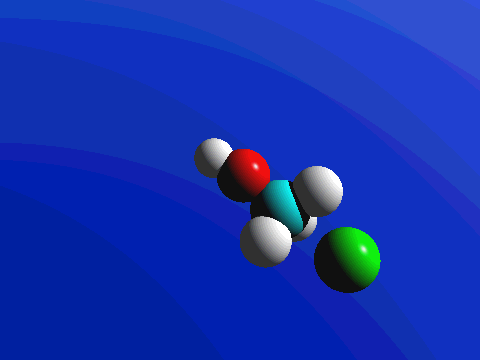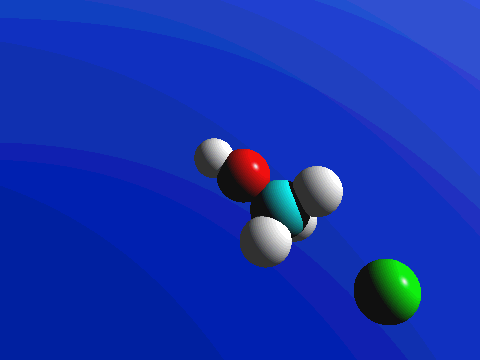
1-Chloro-2,2-dimethylpropane is completely hindered to backside attack.

The only product is the elimination product.
2-Chloropropane is less hindered.

The acetylide ion can attack more readily, so its substitution reaction is faster than on the 3° halide.

