Start with the most stable staggered Newman projection conformer for 3-methylhxane viewed along the #C_3 - C_4# bond
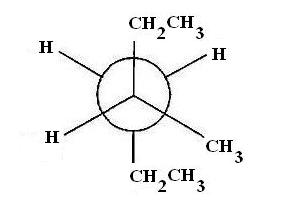
The most stable conformer for 3-methylhexane viewed along the #C_3 - C_4# bond has two ethyl groups in anti position and gauche interaction between an ethyl group and a methyl group.
Let's determine the groups attached to each atom. If #C_3# is the front atom, you'll see that it has attached the ethyl group in the plane of the page, a methyl group coming out of the plane of the page, and a hydrogen atom going into the plane of the page.
The #C_4# carbon is the back carbon and it has attached the ethyl group in the plane of the page, and two hydrogen atoms out of the plane of the page.
Before attaching the groups to each carbon, you must firts draw the parent chain, hexane
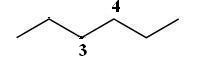
The two ethyl groups are already attached to the #C_3# and #C_4# carbons because thy're part of the parent chain. Since hydrogen atoms are not drawn in bond line notation, the #C_4# carbon will not have anything visible attached to it.
However, the #C_3# carbon will have the methyl group - #CH_3# - attached and visible in the notation.
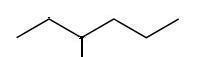
That is the bond line notation for 3-methylhexane.

