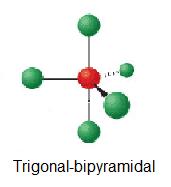
What geometric arrangement of charge clouds is expected for an atom that has five charge clouds ? A) tetrahedral B) trigonal bipyramidal C) octahedral D) square planar
1 Answer
Apr 30, 2015
The answer is D) trigonal bipyramidal.
An atom that is surrounded by 5 regions of electron density will have a steric number equal to 5.
Depending on the value of the coordination number, which expresses the number of atoms with which a central atom forms bonds, you can have the following molecular geometries
The only option that matches is trigonal bipyramidal.