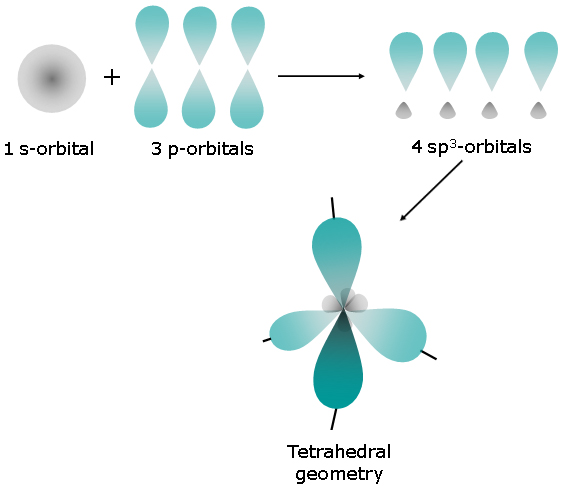
Which orbital hybridization is associated with a tetrahedral charge cloud arrangement ? A) #sp# B) #sp^3d^2# C) #sp^3# D) #sp^2#
1 Answer
May 3, 2015
The answer is C)
Charge clouds, or reagions of electron density, can be bonds (single, double and triple bonds count as 1 region of electron density) or lone pairs.
A tetrahedral charge cloud arrangement thus corresponds to 4 regions of electron density that surround the central atom - this is known as the steric number.
The steric number it also used to determine orbital hybridization, i.e. it gives you the number of hybrid orbitals a central atom must have.
In this case, a steric number equal to 4 corresponds to 4 hybrid orbitals