I do not know what antibonding means and why those types of molecular orbitals are highly energetic. Please explain me with easiest way so that I can understand?
1 Answer
This is based on the amount of nuclear repulsion you get if you form a node within a molecular orbital. The more nuclear repulsions, the greater the energy of the overlap and the less favorable it is.
The linear combination (sum of product terms) of atomic orbitals (AOs) gives molecular orbitals (MO). If for example we take two
#psi_"b" = 1s_A + 1s_B#
#psi_"a" = 1s_A - 1s_B# where
#psi_b# represents the bonding#sigma_"1s"# MO,#psi_a# represents the antibonding#sigma_"1s"^"*"# MO, and#1s# simply represents a#1s# AO.#A# and#B# simply differentiate one#1s# AO from the other.
This can also be pictorially represented as:
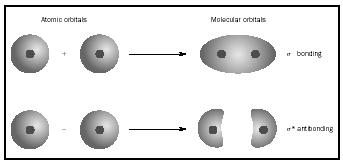
When two AOs overlap in-phase, like for
Normally with a bonding MO, the increased likelihood of electrons appearing in between two atoms promotes the interaction of the nuclei with the electrons, not with each other.
On the other hand, when two AOs overlap out-of-phase, like for
Having a region of no electron density within an antibonding MO means that there can be no electrons for the nuclei to interact with, and so the nuclei interact repulsively with each other, increasing the energy of the orbital overlap.
The more they try to overlap, the greater the nuclear repulsions become and the higher in energy the overall antibonding MO becomes.

