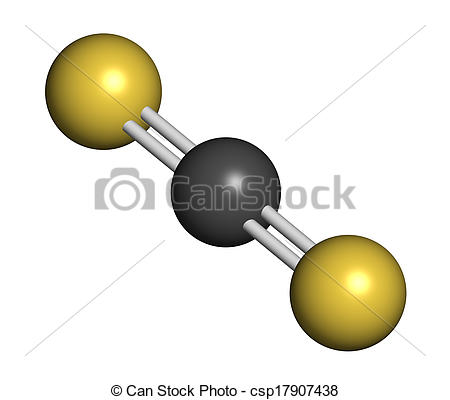
What is the molecular geometry of the CS2 molecule?
1 Answer
Linear.
Explanation:
The best place to start when trying to figure out a molecule's geometry is its Lewis structure.
Carbon disulfide,
The central carbon atom will form double bonds with the two sulfur atoms. These bonds will account for
The remaining
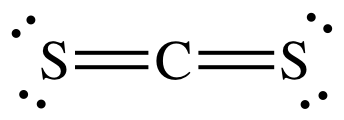
Now, molecular geometry is determined by the hybridization of the central atom. In this case, the carbon atom is surrounded by two regions of electron density, one for each double bond it forms with the sulfur atoms.
This means that its steric number will be equal to
The molecular geometry will thus be linear, the basic