How many sp2 hybridized carbon atoms are present in benzaldehyde?
1 Answer
Seven.
Explanation:
Your tool of choice here will be Lewis structure of benzaldehyde,
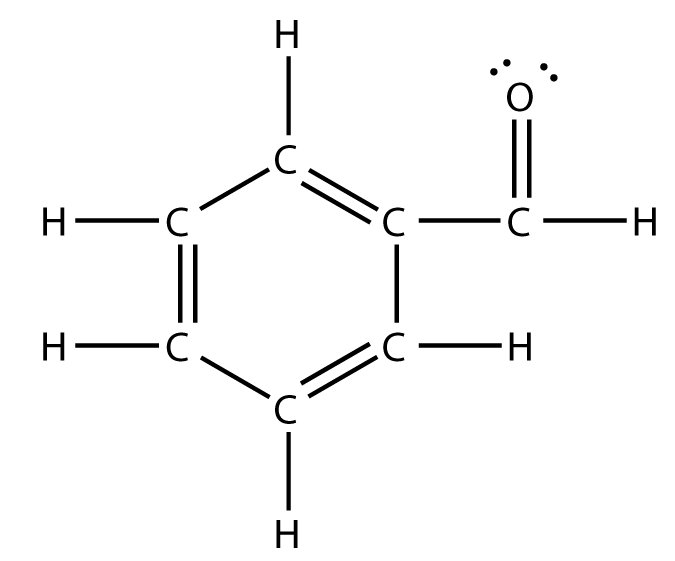
Now, the idea here is that the number of hybrid orbitals will tell you the number of regions of electron density that surround a specific atom - this is known as the steric number.
More specifically, regions of electron density refer to
- single, double, or triple bonds - all three types count as one region of electron density
- lone pairs of electrons - every lone pair of electrons counts as one region of electron density
In this case, an
This means that the atom is surrounded by three regions of electron density. So, take a look at the Lewis structure of benzaldehyde and try to figure out how many carbon atoms are surrounded by that many regions of electron density.
For starters, you know for a fact that you don't have lone pairs of electrons for neither one of those carbon atoms, so all you have to do now is look for how many bonds it forms with other atoms.
As you can see, every carbon atom found in benzaldehyde, meaning all six found in the benzene ring and the one found in the formyl group, forms three bonds with neighboring atoms
- one double bond
- two single bonds
This means that they are all
Here's how the benzaldehyde molecule looks like