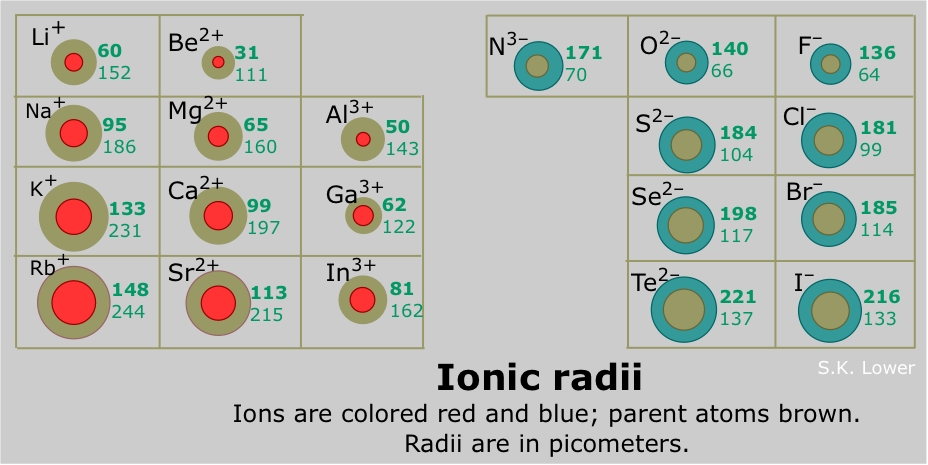
What would be larger, #K# (parent atom) or #K^+#?
1 Answer
Explanation:
Think about what determines atomic size first, then shift focus on applying the same concepts for ionic size.
As you know, the size of an atom is determined by the location of its outermost electrons, that is, the electrons located on the highest energy level.
Simply put, the further away the outermost electrons are from the nucleus, the bigger the atomic size.
As you know, electrons are located in orbitals that correspond to various energy levels. The higher the energy level, the further away from the nucleus the electrons it contains will be.
Potassium,
The electron configuration for a neutral potassium atom looks like this
#"K: " 1s^2 2s^2 2p^6 3s^2 3p^6 color(red)(4)s^1#
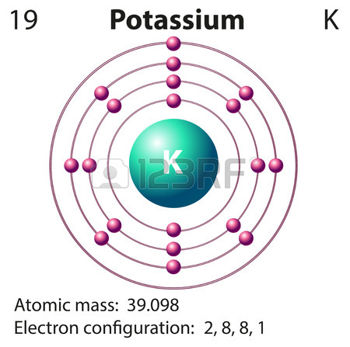
The size of a potassium atom is determined by how far that solitary electron that resides on the highest energy level, i.e on the fourth energy level, is from the nucleus.
Now, the potassium cation,
The electron configuration of the
#"K"^(+): 1s^2 2s^2 2p^6 color(red)(3)s^2 color(red)(3)p^6#
Now the outermost electrons are located on the third energy level, closer to the nucleus. This of course implies that the ion is actually smaller in size when compared with the neutral atom.