What determines an SN1 reaction rate?
1 Answer
If you think about it, in a substitution reaction there really are two main factors that tell you whether it's
Two molecules react, and one displaces a substituent on the other.
All other factors the same, a reaction of this sort can only depend on either or both of these two participants, in some capacity or none.
FACTORS IN SUBSTITUTION REACTIONS
The leaving group propensity is how readily the leaving group breaks its bond with and departs the molecule.
The strength of an incoming nucleophile directly correlates with how readily it approaches the electron-deficient site on a molecule to donate electrons (a nucleophile is a lewis base, i.e. electron donor).
Whichever is more important determines the predominance of
DIFFERENCE BETWEEN SN1 AND SN2
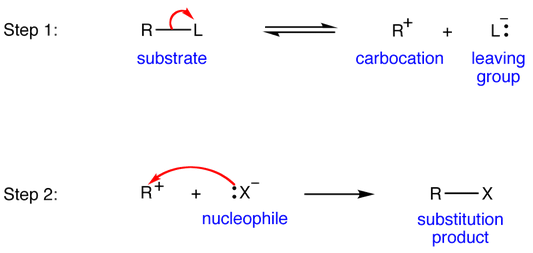
When the leaving group has a weak bond with the electron-deficient site on the molecule, it's going to readily break the bond and leave the molecule without much coercion.
At that point the reaction kinetics depend on the target molecule significantly more than the incoming nucleophile. Thus, it is effectively involving the participation of only one molecule, so we call it
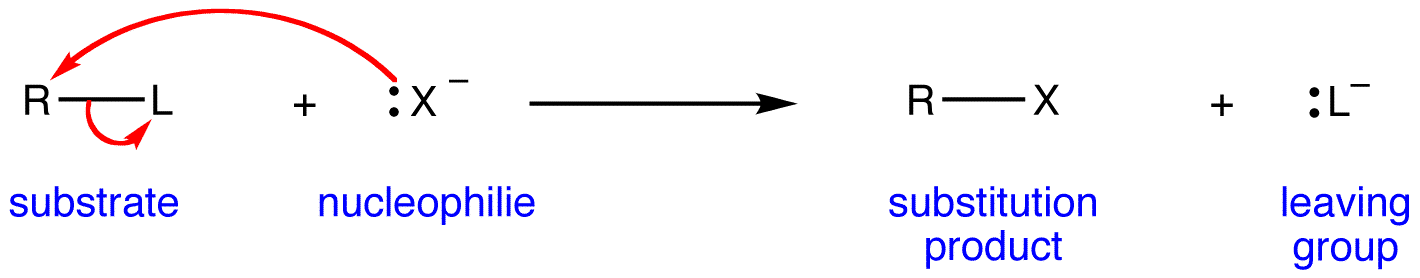
When the nucleophile is strong, it readily approaches and attacks the target molecule with the goal of forming a new non-proton bond.
If the approach of this nucleophile is more important than the molecule breaking a bond with the leaving group, the reaction will effectively involve the participation of both molecules, so we call it

