What is the difference between the trans influence and trans effect on square-planar transition metal complex substitution/interchange reactions? So is the trans influence defined under the trans effect or alongside, or both?
I know that the trans influence is thermodynamic and the trans effect is kinetic, but I think my book is presenting it in a confusing way. Is the trans effect an overall definition, under which the thermodynamic trans effect and kinetic trans influence lie?
It shows that the trans influence raises the energy of the reactants and that the trans effect lowers the activation energy of the transition state.
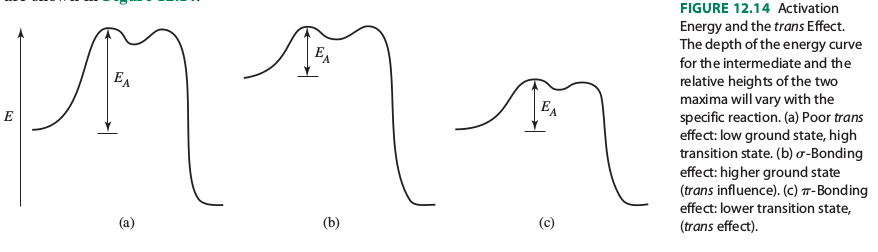
However, it explains these as follows:
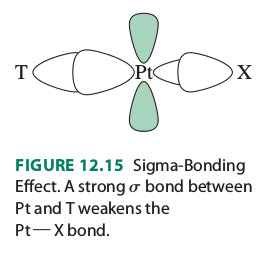
(#T# is the ligand trans to the leaving group #X# )
"The trans effect is rationalized by two factors, weakening of the Pt-X bond and stabilization of the presumed 5-coordinate transition state."
[...]
"The Pt-X bond is influenced by the Pt-T bond, because both use the Pt #p_x# and #d_(x^2-y^2)# orbitals. When the Pt-T #sigma# bond is strong, it uses a larger contribution of these orbitals and leaves less for the Pt-X bond. As a result, the Pt-X bond is weaker, and its ground state (sigma-bonding orbital) is higher in energy. This ground-state, thermodynamic effect is called the trans influence. It contributes to the reaction rate by lowering the activation barrier for Pt-X bond breaking".
[...]
"When the T ligand engages in a strong #pi# -acceptor (backbonding) interaction with Pt, charge is removed from M, rendering the metal center more electrophilic and more susceptible to nucleophilic attack. This is the prerequisite for formation of the 5-coordinate intermediate with a relatively strong [Pt-Nu] bond, stabilizing the intermediate. It is noteworthy that #pi# -backbonding between M and T also stabilizes the intermediate by partially offsetting the increase in energy due to the M-X bond breaking. The energy of the transition state is lowered, reducing the activation energy."
I know that the trans influence is thermodynamic and the trans effect is kinetic, but I think my book is presenting it in a confusing way. Is the trans effect an overall definition, under which the thermodynamic trans effect and kinetic trans influence lie?
It shows that the trans influence raises the energy of the reactants and that the trans effect lowers the activation energy of the transition state.

However, it explains these as follows:

(
"The trans effect is rationalized by two factors, weakening of the Pt-X bond and stabilization of the presumed 5-coordinate transition state."
[...]
"The Pt-X bond is influenced by the Pt-T bond, because both use the Pt
[...]
"When the T ligand engages in a strong
1 Answer
So, I asked my professor about this, and she essentially said:
"Some authors say trans effect vs. trans influence to differentiate between the kinetic and thermodynamic effect, respectively. As a result, saying 'overall trans effect' encompasses both.
Some other authors simply say trans effect for either one, and differentiate by adding the kinetic or thermodynamic qualifier, i.e. trans effect could mean either one without specification."
So it's a matter of how an author has decided to present the information.
Ultimately, there is a kinetic and thermodynamic quality to the impact a ligand trans to the leaving group has on the departure of that leaving group.
For a substitution/interchange reaction between a nucleophile and a square planar transition metal complex, we have this general mechanism, which has not differentiated between the strength of the
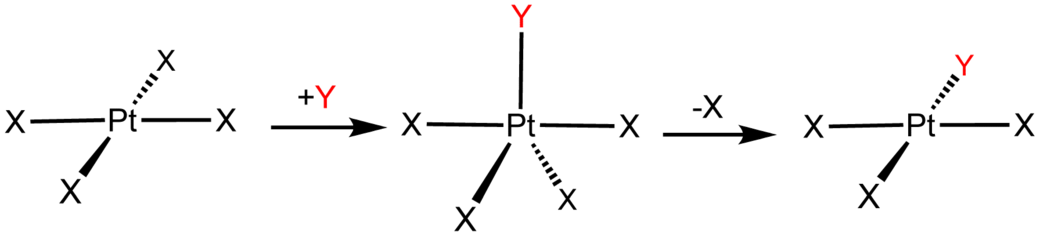
The
THE THERMODYNAMIC FACTOR

If we say the trans influence is thermodynamic, then it describes that:
The stronger the donating interaction of the trans ligand
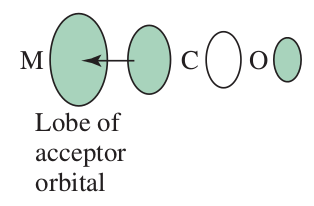
If we say
For instance,
THE KINETIC FACTOR
If we say the trans effect is kinetic, then it describes that:
The stronger the acceptance interaction of the trans ligand
If we say that
For instance,
The combination of a GREATER trans effect (kinetic) and a GREATER trans influence (thermodynamic) respectively makes it easier for the five-coordinate transition state to form and easier for the leaving group to depart.
Miessler et al. came up with the following overall impact of the ligand trans to the leaving group to be in this order:
#"CO ~ CN"^(-)# #"~ C"_2"H"_4 > "PR"_3# #"~ H":^(-) > ""^(-) :"CH"_3# #"~ SC"("NH"_2)_2 > ""^(-) :"C"_6"H"_5 > "NO"_2^(-)# #"~ SCN"^(-)# #"~ I"^(-) > "Br"^(-) > "Cl"^(-) > "pyridine", "NH"_3# #"~"^(-)":OH"# #"~ H"_2"O"#

