Why are single bonds weaker than double?
1 Answer
Simply from how they're constructed. Since a pure double bond consists of 1
All pure single bonds consist of one
Below is an example a constructive
#2p_z-2p_z# head-on overlap that forms a#sigma_(2p_z)# molecular orbital, where electron density lies in the white bulged region---between the atoms.

A nice example is the
#\mathbf(sigma)# bond in#\mathbf("Cl"-"Cl")# .
All pure double bonds consist of an additional
Below is an example of the
#2p_x-2p_x# constructive/bonding overlap, where electron density lies in the white bulged region (above the atoms).
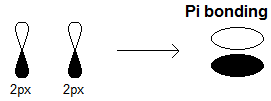
An explicit
#pi# bond example is the#\mathbf(pi)# bond in#\mathbf("O"="O")# , a product of either a#2p_x-2p_x# sidelong overlap, or a#2p_y-2p_y# sidelong overlap (but not both), that forms a#pi_(2p_(x"/"y))# orbital, depending on which pair overlaps.This
#pi# bond is made in addition to the#sigma# bond that was already made upon forming the first#"O"-"O"# bond.
Therefore, since a pure double bond consists of 1

