If I took you literally, no, because two pi bonds in the same triple bond are perpendicular to each other, and that's the only way for two pi bonds to "stack" near each other without electron or nuclear repulsion effects.
They don't overlap at all.
 chemwiki.ucdavis.edu)
chemwiki.ucdavis.edu)
If you meant pi[-compatible] orbitals, then yes, that's how pi bonds can form---through sidelong \mathbf(pi)-compatible orbital overlap .
Two orbitals that overlap sidelong form a pi bond, whether it's two np_x or two np_y orbitals (both lobes overlap), or two nd_(xy) or nd_(xz) or nd_(yz) orbitals (2"/"4 lobes overlap).
Here are examples of sigma bonds (row 1) and pi bonds (row 2):
 useruploads.socratic.org)
useruploads.socratic.org)
 chemwiki.ucdavis.edu)
chemwiki.ucdavis.edu) 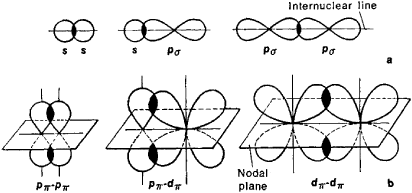 useruploads.socratic.org)
useruploads.socratic.org) 
