Question #3aacd
1 Answer
Explanation:
The blank molecular orbital diagram for

Molecular orbitals for
Each
We use the Aufbau Principle and Hund's rule to place these electrons in the atomic and molecular orbitals.
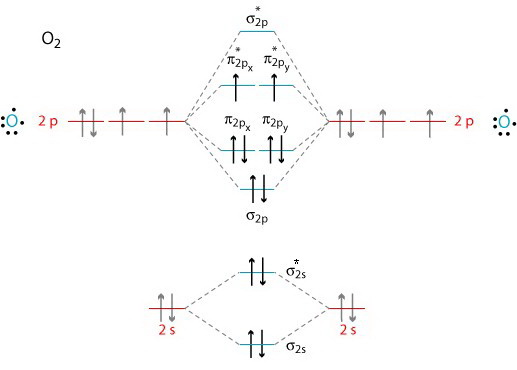
(From www.grandinetti.org)
or
Molecular orbitals for
We remove one electron from the highest energy orbital of

In this diagram,
Molecular orbitals for
We add an electron to the molecular orbital diagram for
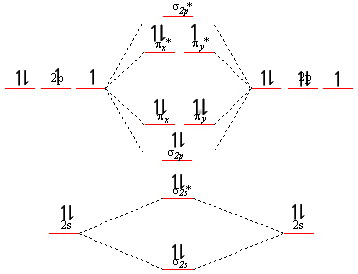
(Adapted from Chemistry - Stack Exchange)

