Why is "Ni"("CN")_4^(2-) diamagnetic but "NiCl"_4^(2-) paramagnetic?
2 Answers
Well is not one square planar, and the other tetrahedral.........??
Explanation:
All of this will be explained much better in your inorganic text; and it will probably give detailed descriptions of splitting diagrams.......
This is due to the former complex being square planar and the latter being tetrahedral. Examine their crystal field splitting diagrams at the bottom of the answer and the supplementary explanation to see where the two unpaired electrons in
DISCLAIMER: LONG ANSWER!
TENDENCIES OF
The difference in energy between these configurations tends to be small. Tetrahedral
REGARDING LIGAND FIELD STRENGTHS
Consider the following diagram for octahedral complexes, which shows the difference in (ligand) field splitting energies for
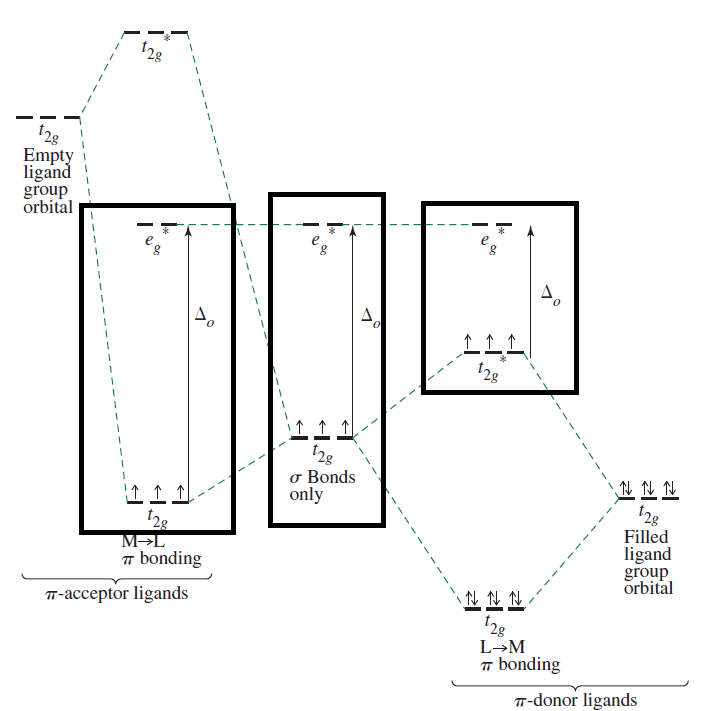
Basically,
pi donors destabilize thet_(2g) orbitals,sigma donors destabilize thee_g^"*" orbitals, andpi acceptors stabilize thet_(2g) orbitals.
In the spectrochemical series,
This further means that...
-
"Cl"^(-) , a weak-field,pi donor ligand, promotes high-spin complexes, since electron-pair repulsions are minimized, giving minimal crystal field energy splitting, and Hund's rule applies for such nearly degenerated orbitals (fill orbitals with one electron at a time, then double up). -
"CN"^(-) , a strong-field,pi acceptor ANDsigma donor ligand, promotes low-spin complexes, since electron-pair repulsions are maximized and the crystal field splitting energy increases significantly. How you fill orbitals for low-spin complexes is to follow Hund's rule for that energy level, allowing electron pairing, then go to the next energy level.
Hence, the chloride complex would favor tetrahedral, and the cyanide complex would favor square planar.
CRYSTAL FIELD SPLITTING DIAGRAMS
Their blank

The
This gives rise to a diamagnetic configuration, as expected.

The
This leaves two unpaired electrons in the


