What is the molecular electron configuration of #"F"_2#?
1 Answer
#(sigma_(2s))^2 (sigma_(2s)^"*")^2 (sigma_(2p_z))^2 (pi_(2p_x))^2 (pi_(2p_y))^2 (pi_(2p_x)^"*")^2 (pi_(2p_y)^"*")^2#
Recall that there are orbital mixing effects for homonuclear diatomic molecules that decrease from left to right until
Notice how the
#sigma_(g)(2p)# , or the#sigma_(2p_z)# molecular orbital, dips down below the#pi# molecular orbital energies after#"N"_2# .
Since
In general, the molecular orbital energies follow these rules:
- The relative atomic orbital energy differences approximate the relative
#sigma//sigma# orbital energy differences.
So,
#sigma_(2s)# molecular orbitals are significantly lower in energy than#sigma_(2p_z)# molecular orbitals because the corresponding#2s# atomic orbitals are significantly lower in energy than the#2p# 's.
#sigma# molecular orbitals are singly-degenerate, and#pi# molecular orbitals are doubly-degenerate.#sigma# molecular orbitals, in principle, get more stabilized upon overlap than#pi# molecular orbitals do.
For example, an
and an
So, the full MO diagram is:
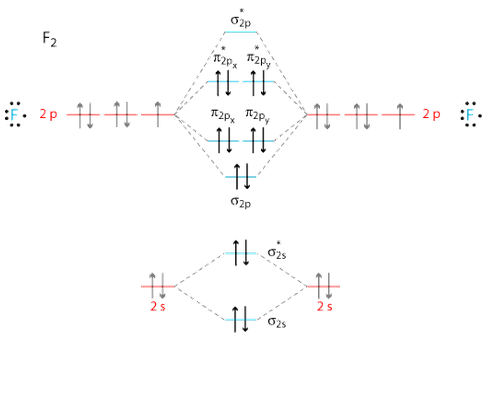
Thus, the valence electron configuration is:
#color(blue)((sigma_(2s))^2 (sigma_(2s)^"*")^2 (sigma_(2p_z))^2 (pi_(2p_x))^2 (pi_(2p_y))^2 (pi_(2p_x)^"*")^2 (pi_(2p_y)^"*")^2)#

