Since #SiO_2# and #CO_3^(2-)# conform to the octet rule the following method can be used to determine the number of covalent bonds for the two structures. If Hydrogen is part of the structure, the valence is '1' and fills with '2' electrons. For elements such as Beryllium or Boron, Valence is 2 and 3 respectively and fill with 4 and 6 electrons respectively. Examples of compounds that have elements that depart from the octet rule are given after #SiO_2# and #CO_3^(2-)#.
#SiO_2# (Silicon Dioxide)
#"Valence" "Electrons" = Si + 2Oxy = 4 + 2(6) = 16#
#"Octet" "Electrons" = Si + 2Oxy = 8 + 2(8) = 24#
Covalent Bonds = #((O_e - V_e)/2)# = #(24 - 16)/2 = 8/2 = 4 Cov Bonds#
#:ddotO=Si=ddotO:# => #2 "double" "bonds"#
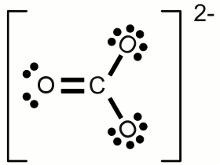
#CO_3^(2-)# (Carbonate Ion)
#"Valence" "Electrons" = C + 3Oxy = 4 + 3(6) + 2 = 24#
#"Octet" "Electrons" = C + 2Oxy = 8 + 3(8) = 32#
Covalent Bonds = #((O_e - V_e)/2)# = #(32 - 24)/2 = 8/2 = 4 Cov Bonds#
#[::ddotO"-(C=ddotO: ) -ddotO::]^(2-)# => 1 double bond 2 single bonds
As examples of a compounds that have elements that do not conform to the octet rule the following is offered...
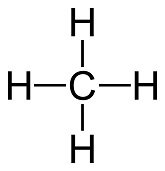
#CH_4# (Methane)
Valence of Hydrogen = 1 and when bonded = 2
Valence Electrons = C + 4H = 4 + 4(1) = 8
Octet/Duet Electrons = C + 4H = 8 + 4(2) = 16
Covalent Bonds = #((O_e - V_e)/2)# = #(16 - 8)/2 = 8/2 = 4 Cov Bonds#
#BeCl_2# (Beryllium Chloride)
Valence = Be + 2Cl = 1(2) + 2(7) = 16
Octet/Duet = Be + 2Cl = 1(4) + 2(8) = 20
Covalent Bonds = #((O_e - V_e)/2)# = #(20 - 16)/2 = 4/2 = 2 Bonds#
(The bonds in #BeCl_2# are more to the ionic bond end of the spectrum than covalent)
#::ddotCl - Be - ddotCl::# ('2' Bonded Pair electrons and '0' non-bonded pair electrons associated with Beryllium. Chlorides have one bonded pair and 3 non-bonded pairs of electrons each.)



