Tungsten has a regular electronic configuration. Being in period #6# and column #6#, we expect it to have a #d^4# configuration and it sure does.
#[Xe] 5d^4 6s^2#
But unlike what you may think, this IS an exception to the Aufbau Principle. Consider the data from Appendix B.9:
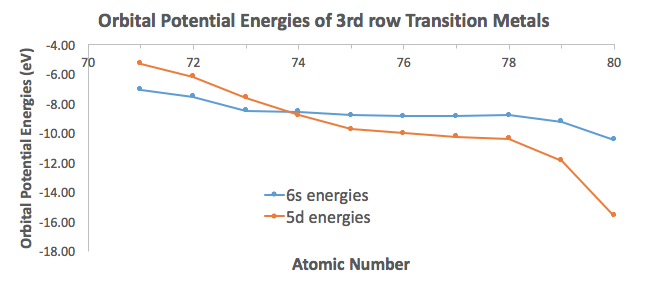
The #6s# orbital of tungsten being slightly above the #5d# orbitals (by about #"0.24 eV"#) makes it so that it is OK for the #6s# electrons to be paired without too much of a loss of stability, even though it goes against the Aufbau Principle to favor pairing an electron in the highest-energy orbital.
The fact that tungsten has this configuration follows from the #5d# and #6s# orbitals being large enough to hold electron density fairly well, which stabilizes the paired electron enough.


