What is the difference between electron shells and electron orbitals?
1 Answer
The dimensionality...?
- The term "electron shells" originates from the Bohr model, a two-dimensional model based on the hydrogen atom only.
It assumes fixed orbits (positions) and fixed trajectories (momenta), and such simultaneous known quantities disobeys the Heisenberg Uncertainty Principle. It also does not identify quantum numbers
#n# ,#l# ,#m_l# , or#m_s# .
- The term "electron orbitals" is part of the modern quantum theory, and is based on a three-dimensional model for any atom.
Such orbitals are regions of electron density, and are related to the probability densities of electron position over allspace, averaged over infinite time.
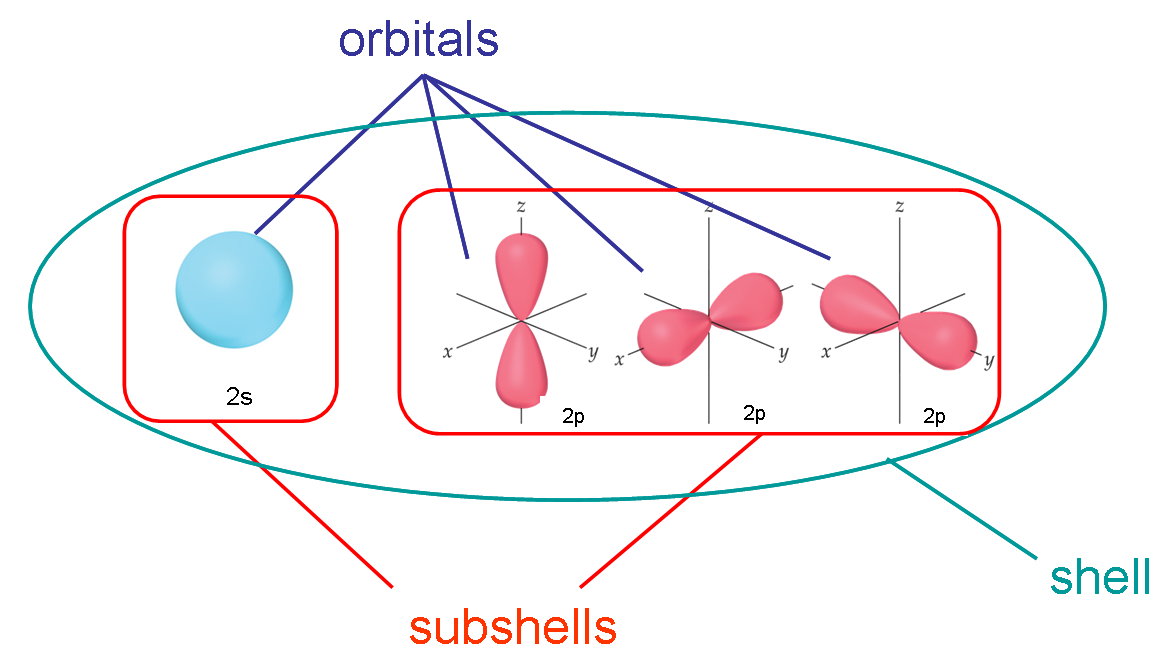
The term "electron shell" lingers on through quantum theory with multi-electron atoms, labeling "shells" as corresponding to each principal quantum number
#n# and "subshells" as corresponding to each angular momentum quantum number#l# .(Electron energy levels do not exist unless the quantum state is accessed, but we still like to think of energy levels as being empty until "occupied".)

