What does single covalent bond mean?
1 Answer
Jun 13, 2018
Just to retire this question...
Explanation:
The modern covalent bond is conceived to be a region of high electron density between two positively charged atomic nuclei, such that electrostatic repulsion between the nuclei is negated and a net ATTRACTIVE force results between the two nuclei and the intervening electron cloud.
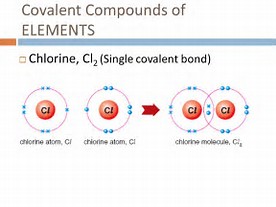
Between the nuclei there is a sweet spot if you like for which attraction between the nuclei to the electron cloud is maximized....and this sweet spot corresponds to HALF of the equilibrium covalent bond-length (why half?). And this is what we mean when covalent bonding is presented as the sharing of electrons between nuclei...

