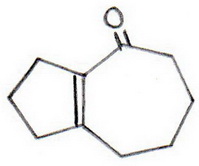
Question #95aae
1 Answer
Jul 3, 2015
This is an intramolecular base-catalyzed aldol condensation.
Explanation:
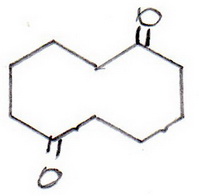
The structure of cyclodecane-1,6-dione is usually written as
But the mechanism will make more sense if we write it as
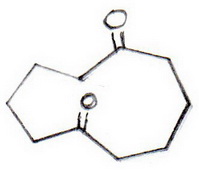
Mechanism
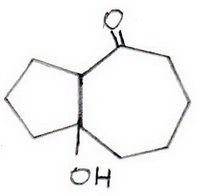
(1) The base removes an α-proton from C-10 (to the left of the top carbonyl group) to form an enolate ion.
(2) The carbanion at C-10 attacks the base of the carbonyl group at
(7-hydroxybicyclo[5.3.0]cyclohexan-2-one).
(3) The aldol is dehydrated under the basic conditions to form an α,β-unsaturated ketone (bicyclo[5.3.0]dec-1,7-en-2-one).