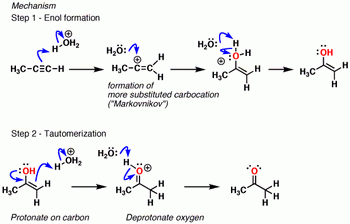
What is the mechanism for the acid-catalyzed hydration of but-2-yne?
1 Answer
See below.
Explanation:
The reaction has two parts:
- Addition of water to the alkyne to form an enol.
- Tautomerization of the enol to form a ketone.
Part 1. Formation of the enol
Step 1. Protonation of the alkyne to form a carbocation.
Step2. Water attacks the carbocation and forms an oxonium ion.
Step 3. Deprotonation of the oxonium to form an enol.
Part 2. Tautomerization of the enol to a ketone
Step 4. Protonation of the alkene carbon atom to form a carbocation.
Step 5. Deprotonation of the oxygen to form the ketone.
If you have trouble following the steps above with condensed structures, here's an image of the same mechanism for the hydration of propyne.