Question #fd54a
1 Answer
Explanation:
You are actually dealing with a reaction deemed responsible for the destruction of Earth's ozone layer.
The starting point for this reaction is the photodissociation of a chlorofluorocarbon molecule in Earth's stratosphere.
Let's take, for example, trichlorofluoromethane,
CFCl3+UV light→CFCl2+Cl
A chlorine atom will then react with an ozone molecule to form chlorine monoxide,
Cl+O3→ClO+O2
Now, chlorine monoxide is a highly reactive compound and will react with anoxygen atom to form oxygen gas and set the chlorine atom loose again
ClO+O→Cl+O2
You're looking for intermediaries and catalysts. As you know, an intermediary is a chemical species that is both formed by the reaction and consumed by the reaction.
For two-step reaction mechanisms, you can identify an intermediary by looking at which species is a product in the first step and a reactant in the second step.
In this case, chlorine monoxide is being produced by the first step, and acts as a reactant for the second step
A catalyst, on the other hand, is a chemical species that increases the rate of the reaction without being consumed by the overall reaction.
For two-step reaction mechanisms, you can identify a catalyst by looking at which species is a reactant in the first step and a product in the second step.
In this case, a chlorine atom matches this description
Now, the overall reaction can be written by adding the two reaction steps. When you do that ,remember to cancel the species present on both sides of the overall chemical equation
{Cl+O3→ClO+O2ClO+O→Cl+O2
----------------------------------------------
Cl+O3+ClO+O→ClO+O2+Cl+O2
Because chlorine is a catalyst, you can write over the reaction arrow. The overall reaction will thus be
O3+OCl×−−→2O2
This reaction has played a major role in the depletion of Earth's ozone layer. The reaction mechanism looks like this
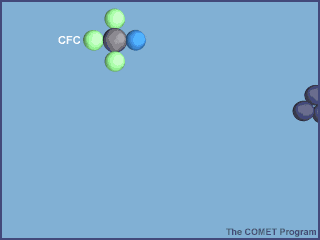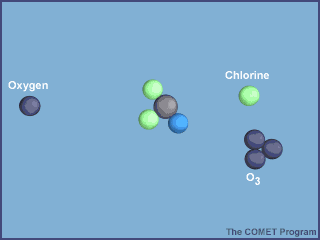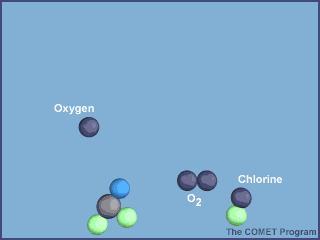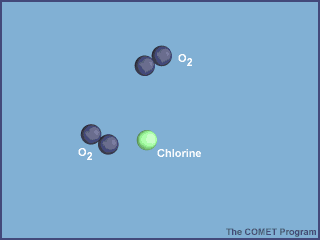
Notice how chlorine monoxide is produced by the first step and consumed by the second, and how the chlorine atom can still be found roaming around after the second step.
In fact, chlorine is such a prolific catalyst for this reaction, that a single chlorine atom can knock out anywhere between
In closing, a very cool video from the great Professor Poliakoff from Periodic Videos

