Help with these reaction questions???
a) "acetylene" -> "2-bromopropane"
b) "1-butene" -> "2,2-dibromobutane"
c) "propyl iodide" -> "2-aminopropane"
d) "acetophenone" -> "2-phenyl-2-propanol"
e) "benzene" -> (Z)"-1,1-methylphenylethene"
f) "bromopropane" -> "2-methylpropan-1-ol"
g) "propene" -> "1,1,2-trimethylethene"
h) "benzyl bromide" -> "phenylacetylene"
i) "ethene" -> "1,1-dimethyl-1-bromopropane"
1 Answer
That's 10 problems but alright. Normally you shouldn't ask 10 questions in one. Next time try asking these separately.
I see you have aromatic reactions, so you can probably do second-semester synthesis.
a) You actually can't just use
Then Lindlar's catalyst catalyzes the partial hydrogenation of the alkyne, but doesn't go all the way (unlike using
b) This one has some complications due to Zaitsev's Rule claiming that the more substituted alkene or alkyne gets generated. It would be great to move the double bond, but there's a better way to do it.
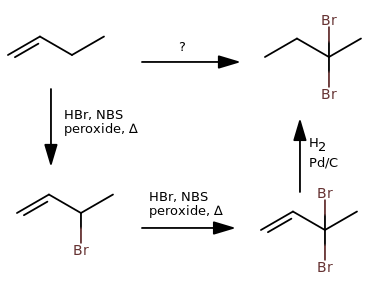
Instead of going through the trouble of brominating, and then eliminating, and then isolating the major product... just use the radical hydrobromination reaction of NBS in the presence of a peroxide and heat, and that gives you a bromine on the allylic carbon.
Do that twice, and you can reduce the double bond last. I found it to save one or two steps.
c) We can get creative with this one. You want a secondary amine, so let's not work with much
This kind of spans both semesters, so it's good review.
Elimination via tert-butoxide to generate a
This can be followed by reacting with ammonia at about
It's probably better than trying to perform
d) You gained one
e) For this one we can get creative and utilize a fun reaction.
The Friedel-Crafts acylation reaction, catalyzed by aluminum chloride, goes through an acylium intermediate before adding on the acyl group. Then the Wittig reaction, which uses a triphenylphosphine compound, basically gives a
f) This one could be done in multiple ways, but here's one that uses some fairly recent reactions for you, some maybe even new.
You've seen the first few steps in part (c). After the Wittig reaction, we can use N-bromosuccinimide, which overall gives bromine at the allylic carbon. Since the alkene was symmetric, it gives good yield.
Then this last bit reduces the double bond, and then replaces the
g) This one was very tricky.
Not a great synthesis, but it works. It aims for a vinylic halide, which can then be used in the Suzuki reaction with an
The NBS reaction adds bromine onto the allylic carbon, which allows for a Gilman reaction in which the alkyl halide is not vinylic or aryl as it would have to be for a Heck or Suzuki reaction.
Gilman works with many types of alkyl halides, and this one qualifies. For it we use a dialkyl lithium cuprate (
The rest you've seen before.
h) This one seems easy, but you cannot just use an acetylide. It makes too many carbons in the end. Gonna see more Wittig action!
i) This one took a lot of thinking... I ended up resorting to alkynes to extend the number of bonds. Then I used HBr with the Suzuki reaction to add in a methyl and the last bromine. Note that using
j) This one might be simple, but I apparently found a pathway using a lot of extensive, sometimes obscure aromatic reactions. Still helps review the reactions in my opinion.
The first step goes through a benzyne intermediate due to the very strong
The nitrile can be turned into a carboxylic acid via heated acid-catalyzed hydrolysis, and the
Finally, the carbonyl can be reduced using

