Question #1ece6
1 Answer
The mechanism shown above is not entirely accurate. It is missing the donation of one of the
THE ACCEPTED MECHANISM
Here is the accepted mechanism:
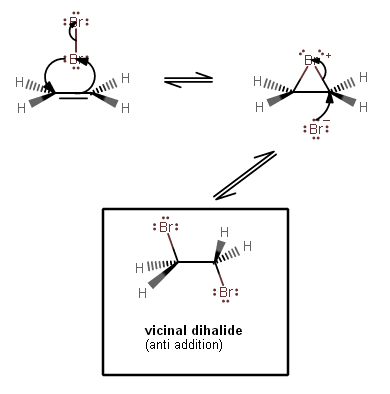
1. The first step should actually have three arrows, which forms a reactive bromonium intermediate, not simply a carbocation intermediate like shown above.
The way the mechanism is written in the question depicts ethene as a nucleophile, when it is supposed to behave as an electrophile (hence, electrophilic addition).
So, the acceptance of electron density into the carbon antibonding orbital is indeed a logical feature that was missing in the mechanism.
The bottom bromine is
#delta^(+)# , and the top bromine is#delta^(-)# , and the latter forms#"Br"^(-)# while the former bonds via approximately one electron per bridging bond as#"Br"^(+)# .
2. The second step then involves
The bromonium bridging bonds are easily broken; you can probably imagine the strain it puts onto the molecule itself. So, the
#"Br"^(-)# becomes a sufficient nucleophile in this step.
DISCUSSION
"I know that the pi bond is an electron dense area where the positively induced dipole on the bromine attacks it"
This doesn't make sense, because a positive dipole doesn't attack anything or donate electron density to anything. It attracts the donation of electrons, or is generated/induced as a result of electron donation.
Bromine is not a nucleophile in this mechanism, though
#"Br"^(-)# can be once the bromonium intermediate forms, because its electron density is "loose" and capably donated.Ethene is the electrophile and nucleophile at the same time, in some sense.
"what i would like to know is why and how this happens in detail."
The bromine is polarized because it is a large and polarizable molecule. Basically, its electron density is "loose" and easily distorted by incoming electron density, such as from ethene.
Because the bond breaks away from the closest bromine atom, that bromine has a positive induced dipole and the remaining one has the negative induced dipole.
The placement of each dipole reflects the final charge acquired by each bromine atom; the negative dipole becomes the full
#-1# charge on the free#"Br"^(-)# ion, for instance.

