Question #425ea
1 Answer
The primary kinetic isotope effect does not affect the reaction mechanism; rather, it gives information on the reaction mechanism.
Explanation:
Kinetic Isotope Effect
The kinetic isotope effect (KIE) refers to the change in rate caused by an isotopic substation in a molecule.
Frequently, the KIE refers to the effect of substituting
The
At ordinary temperatures, most of the molecules are in the
We see the maximum KIE when a
Since
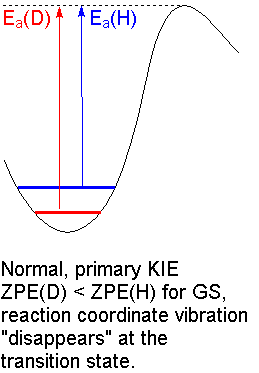
This is called a primary kinetic isotope effect.
The calculated value of
A secondary KIE occurs when the isotope is substituted at a position next to the bond being broken.
Usually, for a secondary KIE,
Mechanistic information from KIEs
KIEs give useful information about the rate determining step in a mechanism.
Case 1
This is consistent with an
Case 2
This is consistent with an

