Question #fbce3
1 Answer
Sep 20, 2017
Four
Explanation:
2,2-dimethylpentane looks like this:
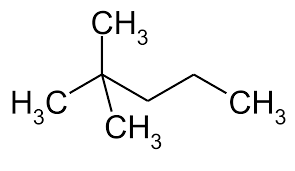
The
1-chloro 2,2-dimethylpentane
Or it may be at C3, C4 or C5
3-chloro 2,2-dimethylpentane
4-chloro 2,2-dimethylpentane
5-chloro 2,2-dimethylpentane
The C2 cannot take the

