Question #026cd
1 Answer
Apr 17, 2017
You get a Wurtz reaction.
Explanation:
The Wurtz reaction is a coupling reaction in which two alkyl halides react with sodium metal in a dry ether solution to form a higher alkane:
The reaction with 1,3-dihalides readily gives cyclopropanes as the product.
Thus, I would expect 1,3-dibromo-2-(bromomethyl)propane initially to form (bromomethyl)cycloprane.
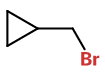
The product is still an alkyl halide.
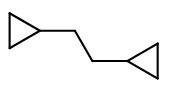
I would expect it to react further and form 1,2-dicyclopropylethane.