Is this how #"CHCl"_2^(+)# acts as an electrophile in the electrophilic aromatic substitution? I feel like I have my mechanism arrows in the wrong direction but I don't know how they should go.
1 Answer
Nope. You can see that
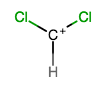
And so, we call
In electrophilic aromatic substitution reactions like these, benzene acts as a nucleophile (a Lewis base), and then restores its aromaticity afterwards.
The electrons should come from benzene first.
(The way you've drawn it suggests you have lost track of two electrons (they ended up on the top middle carbon!), since on the final structure they are not there.)
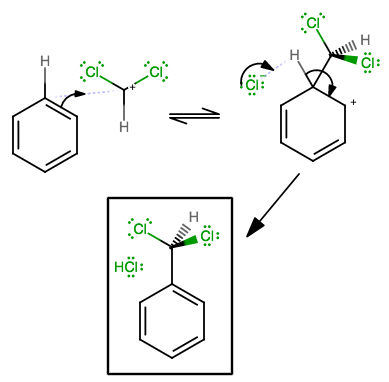
There is only one arrow. It donates away
Then, some base in solution can come to pluck off the


