(a) How will you convert methyl bromide to dimethylamine? (b) How will you prepare propanamine from methyl chloride using suitable reactions?
1 Answer
Here's what I have come up with.
a) While one might expect that it can be accomplished in one step by reacting with methylamine, it would more likely produce a mixture of
Furthermore, it is difficult to control this reaction so that you get to a specific step and stop. So, that isn't the best idea.
However, I actually can't think of any other ideas for this. Since this is probably a theoretical exercise, I guess it's OK to give the following synthesis:
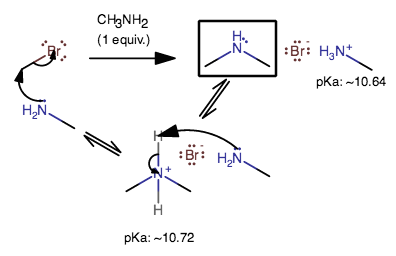
To do this, you may have to increase the pH to be between
You could, however, have a pH above
b) This also asks us to consider reacting amines with alkyl halides. Here's a way to do it without resorting to that.
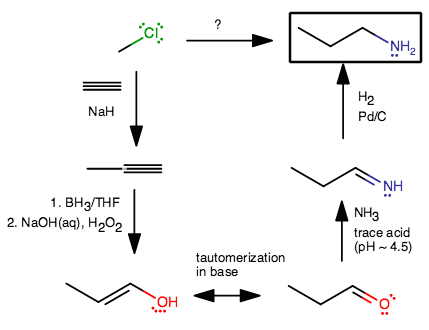
- Using
#"NaH"# would deprotonate acetylene (similar to#"NaNH"_2# ), which can then act as a nucleophile in an#"S"_N2# reaction and form methylacetylene. - Hydroboration adds
#"OH"# onto the less-substituted carbon (anti-Markovnikov) instead of the more-substituted carbon (Markovnikov). - Keto-enol tautomerization occurs to stabilize the terminal enol into an aldehyde.
- Adding ammonia in trace acid (pH near
#4.5# for optimal activity) forms an imine (#"R"-"C"="NH"# , in this case). What happens is that the oxygen gets protonated by the two protons transferred from#"NH"_3# , and leaves as#"OH"_2^(+)# when the tetrahedral intermediate collapses to form the imine. - Adding
#"H"_2# on#"Pd/C"# reduces the#"C"="N"# bond to a#"C"-"N"# bond, similar to how it works on alkenes.

