Considering a Diels Alder reaction with Cyclopentadiene and Maleic Anhydride in ethyl acetate, ligroin - why would the reaction be so hard to occur?
1 Answer
Jul 5, 2016
Just the opposite. You may have to use an ice bath to prevent the reaction from going too fast.
Explanation:
The reaction is
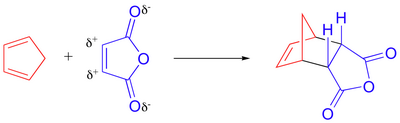
(From chemwiki.ucdavis.edu)
You may have to use heat to dissolve the maleic anhydride in ethyl acetate and then cool the solution slightly before adding the ligroin.
The reaction is exothermic, so you may have to cool the solution in an ice bath before adding the cold cyclopentadiene.
The reaction should take place spontaneously at room temperature, but keep an ice bath nearby in case it goes too fast.

