How do you do addition reactions of alkenes?
1 Answer
May 19, 2016
The first step in the addition reaction, is the interaction between the
The diagram below shows how the reaction takes place and traces the flow of electrons:
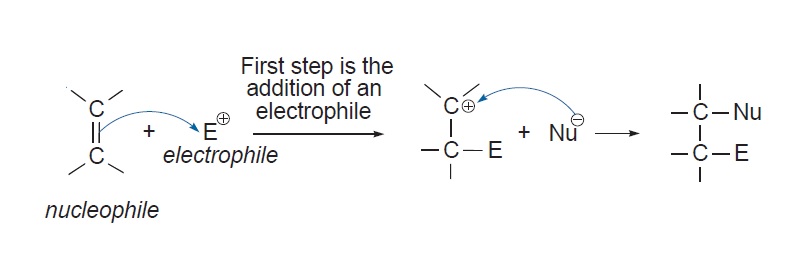
Consider the addition a hydrogen halide
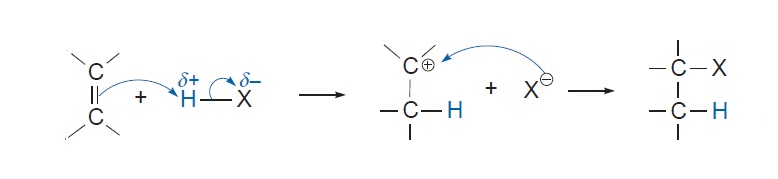
The first step in the addition reaction, is the interaction between the
