How to prepare pentabromobenzene from aniline?
1 Answer
Oct 23, 2016
I can't think of an easy synthesis for this; Ernest Z. suggested more or less what I would have done, but noted that sterics would play into making the yield quite poor.
EDG = electron-donating group
EWG = electron-withdrawing group
wrt = with respect to
EAS = electrophilic aromatic substitution
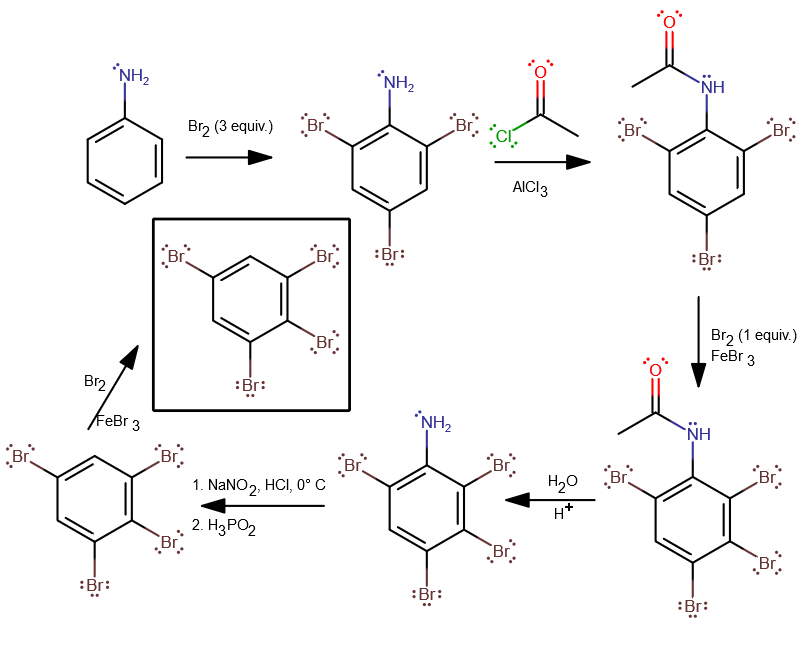
- The amine group is an EDG, so it activates the aromatic ring wrt EAS, allowing the ring to behave as a nucleophile (remember, EAS is where benzene reacts with an electrophile, but isn't an electrophile itself). That means no
#"FeBr"_3# catalyst is required, and the three#"Br"# atoms add easily. - The next
#"Br"# needs to go on meta to the#"NH"_2# , so we have to transform it into a meta-director. One way to do it is to perform a Friedel-Crafts Acylation to generate a protecting group.
This becomes an EWG, which deactivates the ring wrt EAS. It would be a meta-director so that helps. Despite that, having the four EWGs on the ring makes it difficult for another#"Br"# to get on. - We attempt to get a
#"Br"# onto carbon-3 or 5 whichever wants to occur. Either one could occur. This time we do need an#"FeBr"_3# catalyst. - This is the first step to removing the
#"NH"_2# . We wanted to try limiting steric clutter to maximize the chances of a fifth#"Br"# atom making it on. So, we add some acid and hydrolyze the amide bond back into#"NH"_2# and a carboxylic acid side product. - The first step here is the conversion of
#-stackrel(..)("N")"H"_2# into#-stackrel(+)"N"-="N":# , a diazonium cation. The second step removes it completely, using hypophosphorous acid to replace it with an#"H"# . - The last step would be a hopeful
#"Br"# EAS onto one of the remaining carbons. It doesn't matter which one since it's a symmetric molecule.

