Hunsdiecker reaction follows free radical mechanism. Though 1° radical is less stable, the yield of alkyl halide follows 1° > 2° > 3°. Why?
1 Answer
Here's my explanation.
Explanation:
The reaction
The Hunsdiecker Reaction is the reaction of a silver carboxylate with a halogen to form an alkyl halide.
The mechanism
The reaction involves a radical chain mechanism.
Initiation
The bromine reacts with the silver carboxylate (1) to give an unstable acyl hypobromite (2).
The weak
Propagation
The acyl radical loses a molecule of
The alkyl radical reacts with the acyl hypobromite to form an alkyl bromide (5) and generate another acyl radical.
Here's a summary of the steps.
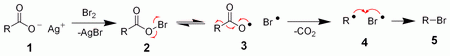
(From Wikimedia Commons)
I would expect the yield of alkyl halide to follow the order 1° > 2° > 3° because
- 1° radicals are the most reactive and
- 1° radicals have the least steric hindrance in the second propagation step (attack on the acyl hypobromite)

