Ortho,para-bromoanisole + #NaNH_2# + Liquid #NH_3# =? How do you predict the product?
1 Answer
Here's how I would do it.
Explanation:
These are the reaction conditions for generating benzyne intermediates.
The reaction of ortho-bromoanisole with potassium amide in liquid ammonia (b. p. -33 °C) is extremely rapid.
Step 1. The amide ion attacks the
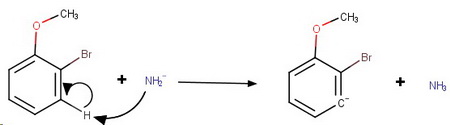
Step 2. Loss of
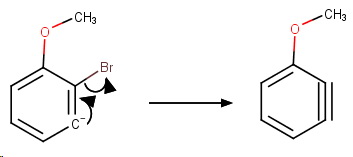
The elimination is by an E2cb pathway.
Step 3. Addition of
The strain caused by a triple bond in a benzene ring can be relieved by a nucleophilic addition (
The methoxy group is electron-withdrawing by induction, so the nucleophile will attack
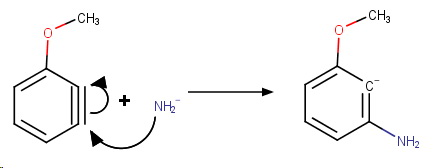
Step 4. Protonation of the carbanion.
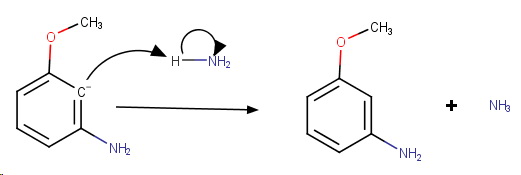
The product is meta-methoxyaniline.
para-Bromoanisole
The reaction with para-bromoanisole also follows a benzyne mechanism.
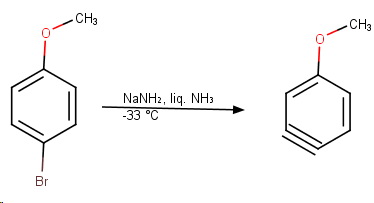
The nucleophile can attack either end of the triple bond, and the methoxy group is far enough away that its inductive effects are minimal.
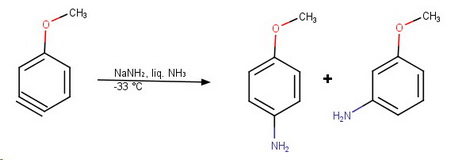
The product is a mixture of para- and meta-methoxyaniline.

