What is a retro Diels Alder reaction?
1 Answer
Feb 3, 2016
It is literally the (microscopic) reverse direction of the Diels-Alder.
A relatively simple but interesting example is the cracking of the cyclopentadiene dimer:
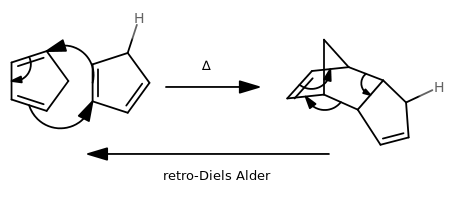
The arrows are pretty much the reverse of what the forward direction shows. Instead of making two
This is a good way, for instance, of generating usually unstable compounds in a rigged fashion such that they are not so unstable.
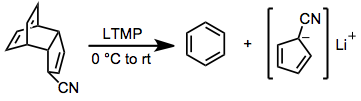
By the way, LTMP stands for lithium tetramethylpiperidide, and "rt" stands for "room temperature". So the example reaction can be done at

