What is the action of hydrogen gas on cyanogen?
Complete the following reactions:
#(CN)_2 + H_2 -> ?#
#(CN)_2 + 4H_2 -> ?#
Complete the following reactions:
1 Answer
Given that a nitrile (
It reduces imines into amines, so it should reduce nitriles (
This parallels the reduction of alkynes (
These types of hydrogenation reactions require a catalyst to cleave the
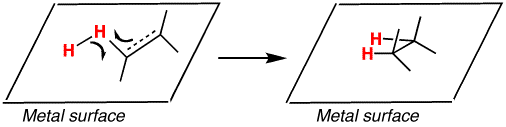
It likely depends on the catalyst whether or not a single
I will assume a palladium catalyst over carbon (as you would traditionally use on alkenes and alkynes for full reduction), and that the catalyst promotes reduction of one
#"N"-="C"-"C"-="N" stackrel("H"_2" "" ")(->) "N"-="C"-"CH"="NH"#
#color(white)(ssaaaaaaaaaaaa)""^("Pd/C")#
#"N"-="C"-"C"-="N" stackrel(4"H"_2" "" ")(->) "H"_2"N"-("CH"_2)_2-"NH"_2#
#color(white)(ssaaaaaaaaaaaa)""^("Pd/C")#
Either way, it accomplishes the same result at each step, just multiple times where applicable.
For the reduction of nitriles, elevated temperatures and pressures promote the reduction.

