What is the Lewis Dot Diagram for Platinum?
1 Answer
A Lewis Diagram serves to represent the number of valence electrons that an element has. Every dot represents a valence electron.
Explanation:
Valence electrons are the electrons in the last layer of an atom. For example, the element Lithium has 1 valence electron. The number of valence electrons increases from left to right in the periodic table. The elements in the last period (row) (example: Xenon) have a full last layer, which means eight valence electrons.
Usually, transition metals such as platinum have 3 valence electrons. However, there are some exceptions. Platinum has only 1 valence electron as the diagram shows below.
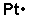
Don't forget to put the atomic symbol (Pt) in this case in the middle. Platinum is not the only element that has only one valence electron (there is sodium, potassium and a few others), so you need to differentiate. It is preferable, unlike the diagram shows, to start at the top of the symbol and work your way around, clockwise.
Practice exercises:
Use the following periodic table to answer the next questions:
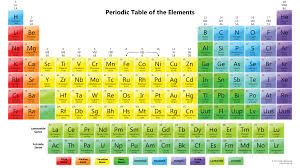
- Find the number of valence electrons in the following elements.
a) Oxygen
b) Radon
c) Boron
-
What element is represented by the following Lewis Dot Diagram?

-
Draw the Lewis Dot Diagram's for the following elements:
a) Strontium
b) Nitrogen
c) Neon
- An element has 7 valence electrons. How many elements fit this criteria?

