What will be product(s) if ethylene glycol is treated with fuming sulphuric acid & heat ?
1 Answer
Dec 9, 2016
Here is my take on it. I anticipate that the alcohol will be protonated on one side, rendering one end of it susceptible to attack by another ethylene glycol. Then, eventually, it may cyclize into 1,4-dioxane.
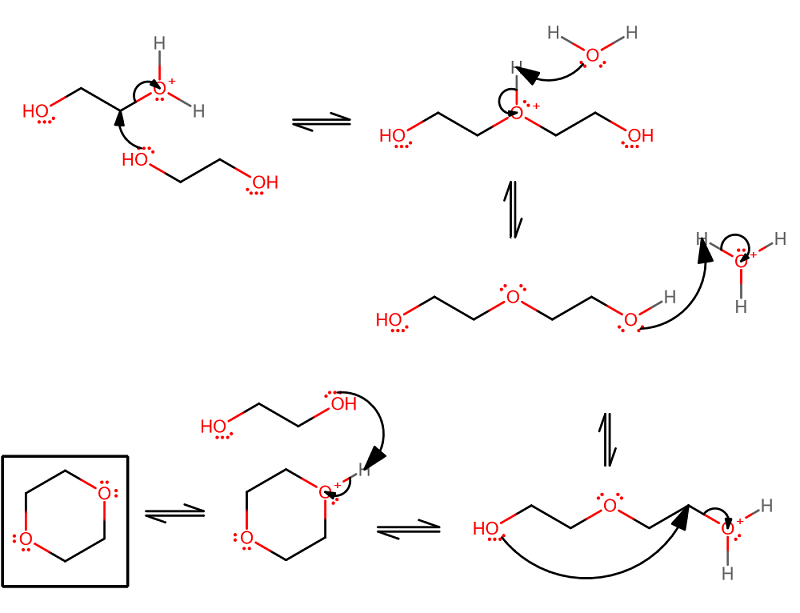
The mechanism is fairly straightforward once you realize that another ethylene glycol can always come in. Another rare possibility is that the ethylene glycol attacks itself and makes an epoxide, but I think that would then get protonated and undo itself anyways...

